ʻO ka maʻi Influenza maloʻo-hau/Maʻi Influenza B waikawa Nucleic
Inoa huahana
HWTS-RT193-Pahu ʻIke Waikawa Nukleika no ka Maʻi Flu/Maʻi Flu B i hoʻomaloʻo ʻia (Fluorescence PCR)
ʻAlapia
Wahi a nā ʻokoʻa antigenic ma waena o nā genes NP a me M, hiki ke hoʻokaʻawale ʻia nā virus influenza i ʻehā ʻano: ka virus influenza A (IFV A), ka virus influenza B (IFV B), ka virus influenza C (IFV C) a me ka virus influenza D (IFV D). No ka virus influenza A, he nui kona mau kikowaena a me nā serotypes paʻakikī, a hiki ke laha ma waena o nā kikowaena ma o ka hoʻohui ʻana o ka genetic a me nā hoʻololi adaptive. ʻAʻohe palekana mau loa o ke kanaka i ka virus influenza A, no laila, he maʻalahi ka poʻe o nā makahiki āpau. ʻO ka virus influenza A ka pathogens nui loa e hoʻoulu ai i nā maʻi ahulau influenza. No ka virus influenza B, laha nui ia ma nā wahi liʻiliʻi a ʻaʻohe ona subtypes i kēia manawa. ʻO nā maʻi kanaka ke kumu nui ʻia e nā virus influenza o B/Yamagata a i ʻole B/Victoria lineages. Ma waena o nā hihia i hōʻoia ʻia o ka influenza i kēlā me kēia mahina ma 15 mau ʻāina o ka ʻāina ʻo Asia-Pākīpika, ʻo ka helu ʻike o ka virus influenza B mai 0 a 92%. ʻAʻole like me ka virus influenza A, ʻo kekahi mau hui o ka poʻe, e like me nā keiki a me ka poʻe ʻelemakule, he maʻalahi i ka virus influenza B, hiki ke hoʻoulu maʻalahi i nā pilikia, e hoʻokau ana i nā kaumaha i ke kaiāulu ma mua o ka virus influenza A.
Nā Palena ʻenehana
| Ka Waihona | 2-28℃ |
| Ola papa | 12 mahina |
| ʻAno Laʻana | ʻUhi ʻāʻī |
| Ct | IFV A,IFVB Ct≤35 |
| CV | <5.0% |
| LoD | 200 Kope/mL |
| Kūlana kikoʻī | ʻO ke kaʻina hana kea: ʻAʻohe kaʻina hana kea ma waena o ka kit a me Bocavirus, Rhinovirus, Cytomegalovirus, Respiratory syncytial virus, Parainfluenza virus, Epstein-Barr virus, herpes simplex virus, varicella-zoster virus, Mumps virus, Enterovirus, Measles virus, human metapneumovirus, Adenovirus, human coronaviruses, novel coronavirus, SARS-CoV, MERS-CoV, Rotavirus, Norovirus, Chlamydia pneumoniae, Mycoplasma pneumoniae, Streptococcus pneumoniae, Klebsiella pneumoniae, Streptococcus pyogenes, Legionella, Pneumocystis jirovecii, Haemophilus influenzae, Bordetella pertussis, Staphylococcus aureus, Mycobacterium tuberculosis, Neisseria gonorrhoeae, Candida albicans, Candida glabrata, Aspergillus fumigatus, Cryptococcus neoformans, Streptococcus salivarius, Moraxella catarrhalis, Lactobacillus, ʻO Corynebacterium a me ka DNA genomic kanaka. Hoʻāʻo hoʻopilikia: Ua koho ʻia ʻo Mucin (60mg/mL), ke koko kanaka (50%), Phenylephrine (2 mg/mL), Oxymetazoline (2mg/mL), Sodium chloride (20mg/mL) me ka mea mālama 5%, Beclomethasone (20mg/mL), Dexamethasone (20mg/mL), Flunisolide (20μg/mL), Triamcinolone (2mg/mL), Budesonide (1mg/mL), Mometasone (2mg/mL), Fluticasone (2mg/mL), Histamine hydrochloride (5 mg/mL), Benzocaine (10%), Menthol (10%), Zanamivir (20mg/mL), Peramivir (1mg/mL), Mupirocin (20mg/mL), Tobramycin (0.6mg/mL), Oseltamivir (60ng/mL), Ribavirin (10mg/L) no ke komo ʻana. hoʻāʻo, a ua hōʻike nā hopena ʻaʻohe hopena hoʻopilikia o nā mea hoʻopilikia ma nā pae i luna i nā hopena hoʻāʻo o ka pahu. |
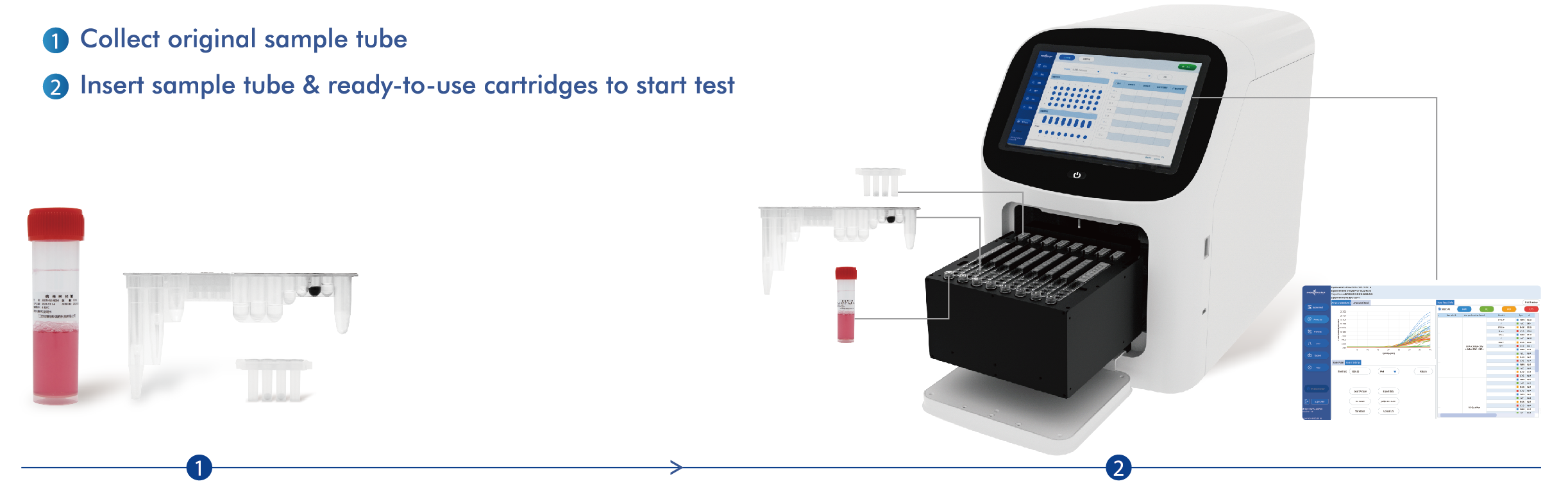
| Nā Mea Hana Pili | Pili i ka mea hoʻāʻo Type I: Nā ʻōnaehana Biosystems i hoʻopili ʻia 7500 Nā ʻōnaehana PCR Manawa Maoli, Nā ʻōnaehana PCR Manawa Maoli SLAN-96P (Hongshi Medical Technology Co., Ltd.). Pili i ka mea hoʻāʻo Type II: ʻO EudemonTM AIO800 (HWTS-EQ007) na Jiangsu Macro & Micro-Test Med-Tech Co., Ltd. |
Kahe Hana
PCR Kuʻuna
ʻO ka Macro & Micro-Test General DNA/RNA Kit (HWTS-3019) (hiki ke hoʻohana ʻia me ka Macro & Micro-Test Automatic Nucleic Acid Extractor (HWTS-3006C, (HWTS-3006B) na Jiangsu Macro & Micro-Test Med-Tech Co., Ltd. ua ʻōlelo ʻia no ka unuhi ʻana i ka hāpana a pono e hana ʻia nā ʻanuʻu ma hope e like me ka IFU o ka Kit.
Mīkini AIO800 āpau-i-hoʻokahi